Science and engineering of carbon aerogel
Release time:2022.02.25
Science and engineering of carbon aerogel
Although carbon aerogel is a unique material with high specific surface area and large mesoporous volume, its supercritical drying cost is very high. Therefore, RF gel was freeze-dried with tert-butanol and carbon gel was thawed by hot cracking. Porosity of carbon jelly. The Vmes value of cold gels is about 60-120% of that of carbon aerogel. Carbon jelly is a kind of high specific surface area & GT; 800m2G-1, large mesoporous volume > 0.55 cm3G-1 mesoporous material.
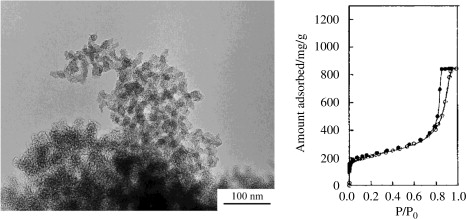
Mesoporous carbon aerogel was prepared from the pyrolysis of resorcinol and formaldehyde. In the figure, TEM images of carbon aerogel with an apparent density of 0.4g/cm3 are shown. The primary carbon particle size is about 4-9nm, forming a network structure. Typical adsorption isotherms of carbon aerogel are shown in the figure, which belong to type IV and have obvious hysteresis. The pore structure parameters calculated by the αs diagram are listed on the three carbon aerogels prepared from resorcinol and formaldehyde at 1000℃, as shown in the table. These carbon aerogels mainly contain mesopores, which are formed in a three-dimensional network of interconnected tiny carbon particles, with only a few micropores forming in the primary carbon particles.
At 900°C, carbon dioxide can activate carbon aerogel to increase Vmicro. Activation increased micropores and mesopores: micropores were 0.68cm3/g, mesopores were 1750m2/g, mesopores were 2.04cm3/g, and mesopores were 510m2/g. The adsorption of nitrogen at 77k and water vapor at 303k on activated carbon aerogel with zero surface functional groups was studied. It is clear that the amount of adsorbed water mainly corresponds to Vmicro, not Vmeso. The addition of Ce and Zr to carbon aerogel produces microporous carbon.
The change of pore parameters of carbon aerogel after high temperature heat treatment is shown in the table. With the increase of HTT, both the total specific surface area and volume decrease, which is mainly due to the decrease of micropores. Therefore, carbon aerogel containing only mesoporous carbon can be obtained by heat treatment above 2000℃.
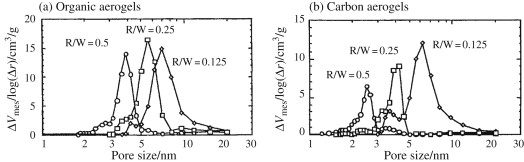
The pore structure of carbon aerogel was controlled by the pore structure of precursor organic aerogel, which was controlled by the mole ratio of resorcinol to formaldehyde (R/F), water (R/W) and sodium carbonate base catalyst (R/C). The synthetic hydrogel was dried with carbon dioxide under supercritical conditions. In the figure, the pore size distribution of both the original organic gel and the generated carbon gel are functions of R/W, and other controlling factors R/F and R/C are 0.5 and 75, respectively. The pore size distribution of organic aerogel is obvious, and its maximum value decreases with the increase of R/W ratio. By carbonizing these organic aerogels, the pore size distribution is shifted to a smaller size, mainly due to the shrinkage of the gel during thermal decomposition.
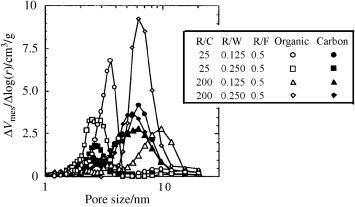
Freeze-drying also replaced supercritical drying of water-based gels. On gels prepared by freeze-drying (freeze gels), less pore shrinkage during carbonization was observed, as shown. The preparation of resorcinol-formaldehyde gel by sol-gel condensation and the preparation of resorcinol-formaldehyde gel by freeze drying were studied in detail to control the mesoporous properties of synthetic carbon materials. Resorcinol formaldehyde gel was prepared by freeze-drying, microwave drying or hot air drying of hydrogel, and heated to carbon gel at 1000℃. In order to obtain mesoporous carbon, the first two drying methods are effective.
The mesoporous carbon aerogel has the possibility of forming reaction field and can be used as template for the preparation of new materials. High crystal zeolite with homogeneous mesoporous channels (ZSM-5 and Y) was prepared using carbon aerogel as template.







